Beauty Product Certifications Explained (FDA, CPNP & More)
If you’re sourcing or selling beauty and cosmetics products, certifications are not optional — they’re what protect your business from shutdowns, fines, seized inventory, or worse.
This guide explains exactly which beauty certifications matter, what suppliers should provide, and what you are responsible for — without legal jargon or scare tactics.
Whether you’re selling wholesale cosmetics or launching a private label brand, this article will help you stay compliant in 2026.
Why Beauty Product Certifications Matter
Beauty products come into direct contact with skin, hair, lips, and eyes. That makes them high-risk products in the eyes of regulators.
Certifications help ensure:
Product safety for consumers
Legal compliance in your selling country
Protection for you as the seller or brand owner
Ignoring certifications is one of the most common (and expensive) mistakes I see new beauty founders make.
FDA (United States)
What is the FDA?
The Food and Drug Administration (FDA) regulates cosmetics sold in the United States.
Important truth (many people get this wrong):
The FDA does not pre-approve cosmetics before they’re sold.
Instead, it requires that products:
Are safe for use
Are properly labeled
Do not contain prohibited ingredients
Are manufactured under sanitary conditions
Who is responsible?
Wholesale sellers: You must ensure the product is FDA-compliant
Private label brands: You are legally responsible for compliance
What to check:
Ingredient list (INCI names)
Proper labeling (manufacturer, distributor, country of origin)
No medical or drug claims
👉 If you sell in the U.S., FDA compliance is non-negotiable.
Related Articles
CPNP (European Union)
What is CPNP?
The Cosmetic Products Notification Portal (CPNP) is the EU’s official system for notifying cosmetic products before sale.
Every cosmetic product sold in the EU must be registered in the CPNP.
What’s required:
Product formula details
Safety assessment by a qualified assessor
Responsible Person (EU-based)
Packaging and labeling details
Who is responsible?
If you’re private labeling: YOU (or your appointed Responsible Person)
If you’re reselling wholesale EU brands: The brand usually handles this — but you should verify
👉 No CPNP = product cannot legally be sold in the EU.
CPSR (Cosmetic Product Safety Report)
What is a CPSR?
A Cosmetic Product Safety Report evaluates whether a cosmetic product is safe for human use.
It is mandatory for the EU and often requested by serious distributors elsewhere.
Includes:
Ingredient toxicity analysis
Exposure assessment
Manufacturing review
Safety conclusion
Who needs it?
Private label brands
Importers bringing cosmetics into the EU
👉 If a manufacturer can’t provide or support a CPSR, that’s a red flag.
MSDS / SDS (Material Safety Data Sheet)
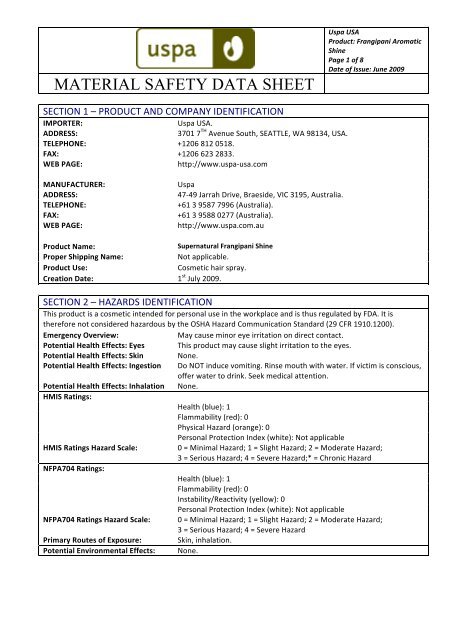

What it is:
An MSDS (now commonly called SDS) provides safety information about chemical ingredients.
Why it matters:
Required for shipping (especially air freight)
Helps with customs clearance
Used for workplace safety
Common misconception:
MSDS ≠ cosmetic approval
It’s a supporting document, not a compliance certificate.
GMP (Good Manufacturing Practices)
What is GMP?
GMP ensures products are made in clean, controlled, and traceable environments.
Common standards:
ISO 22716 (cosmetics-specific GMP)
General GMP certification
Why it matters:
Reduces contamination risk
Builds trust with distributors and retailers
Often required for private label contracts
👉 Reputable manufacturers should already follow GMP.
COA (Certificate of Analysis)
What it is:
A document confirming that a specific product batch meets quality and safety specifications.
Useful for:
Verifying consistency
Quality control
Import documentation
Not always mandatory, but very helpful.
Who Is Responsible for Compliance? (This Is Critical)
If you sell wholesale cosmetics:
The brand handles most certifications
YOU must still verify compliance for your market
If you private label cosmetics:
You are legally responsible
Even if the manufacturer “provides documents”
👉 Never assume a supplier has handled everything.
Common Certification Mistakes to Avoid
Assuming FDA “approval” exists for cosmetics
Selling EU products without CPNP registration
Trusting certificates without verification
Using vague claims like “100% safe” or “medical grade”
Selling imported products without checking local regulations
These mistakes are completely avoidable with the right guidance.
My Practical Advice (From Real Sourcing Experience)
If you’re just starting:
Begin with wholesale brands already compliant
Learn the basics before private labeling
If you want to private label:
Work with manufacturers experienced in your target market
Budget for compliance early
Don’t rush product launches
Compliance is not a barrier — it’s a filter that protects serious founders.
Want Help Finding Compliant Beauty Suppliers?
If you’d rather not figure this out alone:
I’ve curated verified beauty & cosmetics suppliers
I help founders source manufacturers familiar with FDA & EU requirements
👉 Explore the Beauty & Cosmetics Suppliers Directory
👉 Request personalized sourcing support
Your Next Step
Ready to start selling without feeling awkward?
You don’t have to stress about what to post — or struggle to look professional online.
📌 Upgrade Your Content: Get my Instagram Product Templates for E-commerce and start posting scroll-stopping visuals today.
👉 Grab Templates Here →
📌 Source With Confidence: Find products your audience actually wants to see on TikTok & Instagram with my Cosmetics Wholesalers List — 150+ vetted suppliers worldwide.
👉 Get the Supplier List
📌 Stay Ahead of the Trends: Join my weekly newsletter for beauty business owners — get supplier updates, marketing tips, and sales strategies straight to your inbox.
👉 Sign Up Here →


